FDA 510(k)
Laser Hair Brush
Why is an FDA 510(k) Important?
In the United States and abroad, thousands of devices are developed and placed on the market each year, claiming case studies and medical status. However, many of these claims are backed up with little more than words. In order to protect people from false claims and dangerous devices, the United States requires stringent and rigorous testing and proof in the form of a 510(k) submission to the FDA before being granted clearance to be sold to the public. This testing includes clinical studies and laboratory data provided by the applicant. This data must meet specific requirements and is then reviewed by experts within, and sometimes, outside of the FDA. Only after gaining approval by these experts is an FDA 510(k) cleared status given to a device. The purpose of this submission is to prove the SAFETY and EFFICACY of a Medical Device.
After years of research and development, Sunetics International received it's FDA 510(k) clearance for it's Laser Hair Brush. Three different models of the Laser Hair Brush were granted FDA 510(k) clearance. The Sunetics Laser Hair Brush was cleared to GROW HAIR and treat hair loss in both men and women on June 27th, 2013.
Sunetics International hand-held Laser Hair Brush has been granted the
following FDA 510(k) number: K121920


The Indications of Use apply to the Sunetics Clinical Laser Unit AND the Sunetics Laser Hair Brush
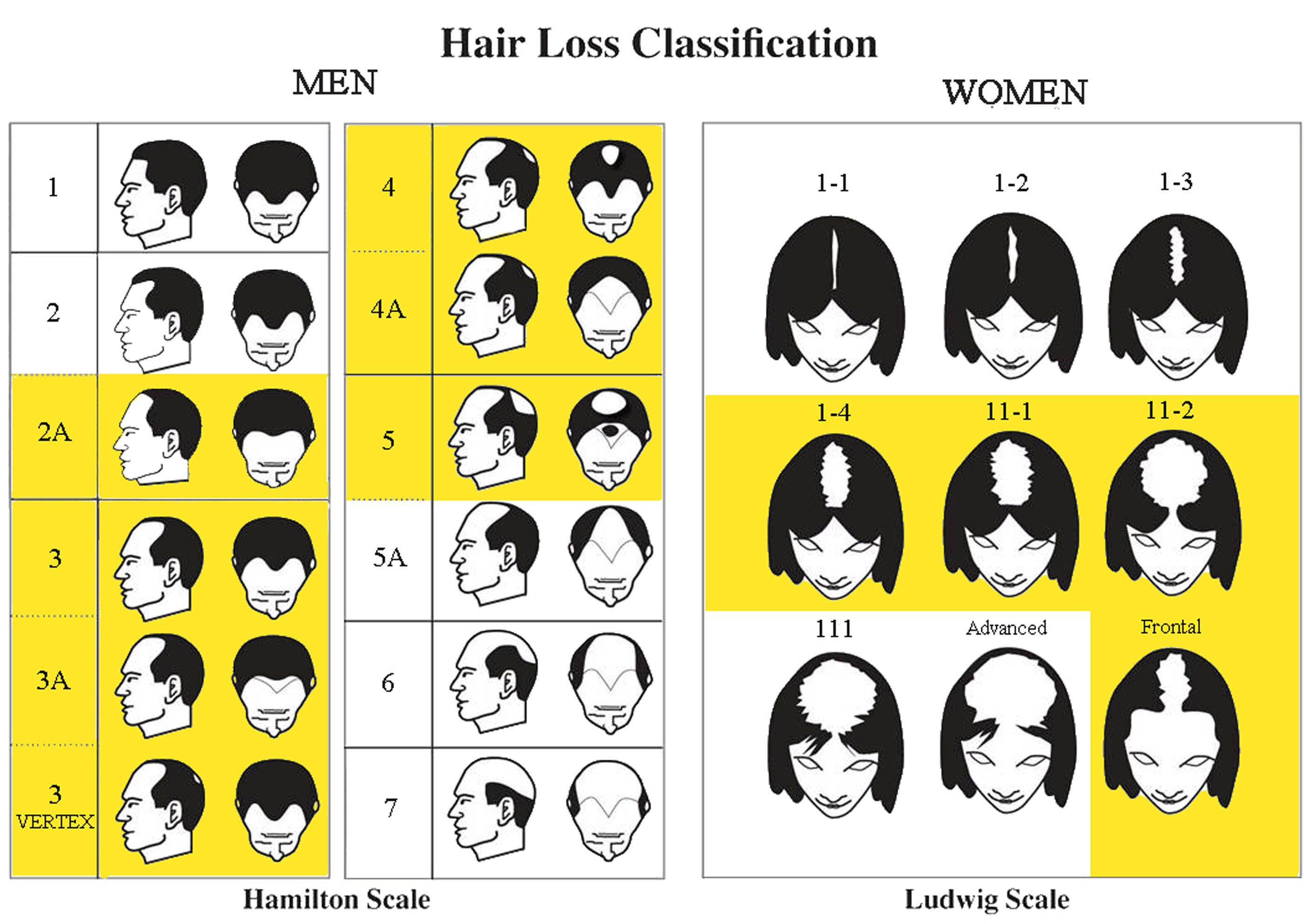
INDICATIONS OF USE
The Sunetics Laser Technology is indicated to treat Androgenetic Alopecia (Hair Loss) and to promote hair growth in Males who have Norwood Hamilton Classifications of IIa to V and Fitzpatrick Skin Types I to IV.
The Sunetics Laser Technology is indicated to treat Androgenetic Alopecia (Hair Loss) and to promote hair growth in Females who have Ludwig (Savin) I-4, II-1, 11-2, or frontal patterns of hair loss and Fitzpatrick Skin Types I to IV.



